The Anatomy of a Lithium-Ion Battery
To appreciate a battery's safety, it is important to know its internal structure. Any lithium-ion battery works because four key parts interact precisely. Together, they create a controlled chemical reaction to produce power.
The Core Components: Cathode, Anode, Electrolyte, and Separator
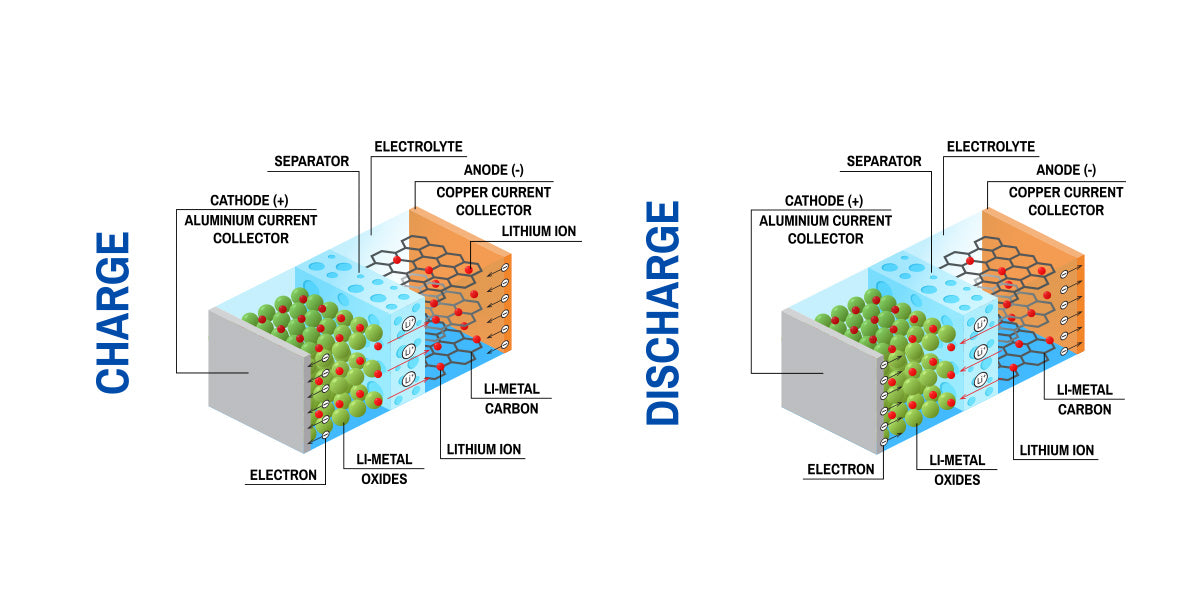
Every lithium-ion battery cell is built from four key components. The cathode is the positive electrode, and the anode is the negative electrode. These two electrodes are materials that store lithium atoms. The cathode material is very important because it defines the battery's voltage, capacity, and safety.
A liquid chemical solution, known as the electrolyte, fills the space between the electrodes. Its job is to help lithium ions move from one electrode to the other. The final component is the separator, a porous membrane that creates a physical barrier between the cathode and anode. This barrier is very important to prevent direct contact. Direct contact would cause a short circuit.
The Flow of Energy: How Ions and Electrons Power Devices
Electricity is made by a controlled movement of particles. During discharge, when a battery is powering a device, lithium ions travel from the anode to the cathode through the electrolyte. Also, electrons are released from the anode. These electrons are unable to pass through the separator, so they travel through an external circuit connected to the device. This flow of electrons is the electrical current that powers the device.
When the battery is charged, an external power source applies a voltage. This reverses the flow. Lithium ions are driven from the cathode, back through the electrolyte, and are stored in the anode again.
The separator's role is more than just a passive divider. It is an engineered safety component. In many common lithium-ion batteries, the separator is made from a polyolefin material like polyethylene. This material is designed to act as a thermal fuse. If the cell's temperature gets too high, around 130°C, the separator material melts. The melting action closes the microscopic pores within the membrane. This pore closure stops the movement of lithium ions between the electrodes, which shuts down the battery's electrochemical reactions. The shutdown is a built-in safety feature. It is designed to stop overheating before it becomes more dangerous.
Understanding the Hazard: The Mechanism of Thermal Runaway
Batteries have internal safeguards, but some conditions can overwhelm them. This can lead to a dangerous failure. This process is called thermal runaway. It is an uncontrolled chain reaction that can cause a fire or explosion. This is a major safety concern for lithium-ion technology.
What Triggers a Battery Failure?
Thermal runaway can start from several types of abuse or failure. External factors include mechanical damage, like a puncture or crushing from an impact. This can cause internal layers to touch. Electrical abuse, like charging a battery beyond its maximum voltage or creating an external short circuit, also creates too much heat. High outside temperatures can also make a cell unstable.
Internal failures are another big cause. Manufacturing defects, such as microscopic contaminants or misalignments inside the cell, can create a path for an internal short circuit to develop over time. No matter the trigger, all these events create a small spot of intense heat inside the battery. If this heat builds up faster than the battery can release it, a self-sustaining reaction starts.
A Cascade of Reactions: The Four Stages of Catastrophe
Thermal runaway is not a single event. It is a rapid cascade of heat-releasing reactions. The process usually happens in clear stages as the internal temperature goes up.
- Around 80°C: The first stage involves the breakdown of a protective layer on the anode called the Solid Electrolyte Interphase (SEI). When this layer fails, it exposes the very reactive anode material to the electrolyte. This starts chemical reactions that create heat.
- Around 100°C: As the temperature increases, the electrolyte itself begins to break down. This releases flammable hydrocarbon gases, which increases the internal pressure of the cell.
- Around 130°C: The separator melts, as it is designed to do. But in a runaway situation, the heat is already too great. The separator's collapse allows the cathode and anode to come into direct contact. This causes a massive internal short circuit that creates a huge burst of heat very quickly.
- Above 150°C: The cathode material was stable at lower temperatures. Now it becomes unstable and starts to break down structurally.

Each stage in this process creates more heat. This heat then speeds up the next stage. This dangerous feedback loop is the main characteristic of thermal runaway. Once it starts, it is an uncontrollable process that keeps itself going.
The Role of Oxygen in Fueling the Fire
The final stage is cathode decomposition. It is the most critical and dangerous part of the process. The initial stages create heat and flammable gas. But the decomposition of conventional cathode materials like Lithium Nickel Manganese Cobalt Oxide (NMC) or Lithium Nickel Cobalt Aluminum Oxide (NCA) releases a key ingredient: oxygen.
When oxygen gas is released into a sealed, hot battery cell already filled with flammable electrolyte vapors, it creates a dangerous and explosive mixture. Research shows that the strong reaction between this released oxygen and the electrolyte triggers the biggest temperature spike and the final, catastrophic failure. The presence of both fuel and an oxidizer inside the cell provides all the parts needed for a fire. So, preventing the cathode from releasing oxygen is the biggest challenge in designing a safe lithium-ion battery.
The LiFePO4 Solution: A Fundamentally Stable Cathode
The great safety of Lithium Iron Phosphate (LFP) batteries comes directly from the special chemistry and structure of their cathode material. This material, LiFePO₄, is naturally stable. This stability directly fights the failure types seen in other lithium-ion chemistries.
The Olivine Crystal Structure: A Fortress for Atoms
The LiFePO₄ cathode material has an olivine-type crystal structure. This is a molecular arrangement known for being very strong. Within this structure, iron (Fe) atoms are held in octahedral arrangements (shown as FeO6 ), and phosphorus (P) atoms are in tetrahedral arrangements (shown as PO4 ). These units are linked together. They form a rigid and stable three-dimensional framework.
This framework is completely different from the layered structures found in NMC and NCA cathodes. In the olivine lattice, the FeO6 octahedra are physically separated from one another by the PO4 tetrahedra. This separation is important because it stops a continuous network from forming. Heat could easily spread through such a network. The structure itself acts as a barrier to the spread of heat, containing it at the atomic level.
The Power of the Phosphate Bond
The key to LFP's great thermal stability is the phosphate (PO4 ) group. The bonds between the phosphorus atom and the four oxygen atoms are covalent bonds, which are very strong. These powerful bonds lock the oxygen atoms tightly within the crystal lattice.
So, the LFP cathode is very resistant to breaking down, even at very high temperatures. It does not begin to break down and release its oxygen atoms until it is heated above 400°C, and sometimes over 500°C. This feature directly prevents the most dangerous stage of thermal runaway. Other cathode chemistries fail and release oxygen around 150-200°C. But the LFP cathode stays structurally and chemically whole. This keeps a potential fire from getting the oxygen it needs to start.
Structural Integrity During Use
Besides being thermally strong, the LFP structure is also mechanically stable during use. As lithium ions move into and out of the cathode during charging and discharging, the olivine framework changes very little in volume, typically less than 7%. The material just changes between its lithiated state (LiFePO4 ) and its delithiated state (FePO4 ). Both states have a similarly stable structure.
But other cathode materials like Lithium Cobalt Oxide (LCO) can expand and contract a lot during cycling. Over thousands of cycles, this repeated mechanical stress can cause tiny cracks to form in the cathode material. These cracks can degrade the battery's performance and create possible sites for internal short circuits to develop. LFP's structural stability means it is highly resistant to this type of mechanical fatigue. This resistance helps give it a very long cycle life and long-term safety. So, the safety of LFP is not just one feature. It is a combination of defenses. It has a chemical defense from its strong P-O bonds, a structural defense from its separated octahedra, and a mechanical defense from its resistance to cycling fatigue.
A Direct Comparison: LFP Safety Versus Other Chemistries
The better safety of LFP is not just a theory. It is shown clearly in lab tests. When put under abuse conditions, the difference in behavior between LFP and other common lithium-ion chemistries is not small, but huge.
The Critical Difference: Oxygen Release Temperatures
The most important safety difference is the temperature at which the cathode becomes unstable. As we have seen, LFP cathodes keep their structure and hold their oxygen atoms until temperatures go above 400°C. But high-energy cathodes like NMC and NCA have layered crystal structures and are much less stable. They begin to break down and release oxygen at much lower temperatures, typically in the range of 150°C to 200°C.
This wide temperature gap is a basic difference in safety. LFP chemistry provides a massive thermal buffer. It remains stable far beyond the point where other chemistries have already begun the irreversible and dangerous process of thermal runaway. That process is fueled by their own oxygen release.
Evidence from the Laboratory: Calorimetry Test Results
Accelerating Rate Calorimetry (ARC) is a testing method that simulates a worst-case thermal runaway scenario inside a controlled instrument. It measures the maximum temperature and the maximum rate of temperature increase a battery experiences during failure. The results from these tests give clear, numerical proof of LFP's better stability.
The data in the table below compares four common cathode chemistries under identical ARC test conditions. The values for maximum temperature (Tmax ) and the normalized maximum heating rate (HRmax, nominal ) show how bad the thermal runaway event is for each chemistry.
Thermal Runaway Characteristics of Common Cathode Chemistries
| Cathode Chemistry | Max Temperature Reached (°C) | Max Heating Rate (°C min⁻¹ Ah⁻¹) | Danger Level Ranking |
| LFP (LiFePO4 ) | 239.26 | 0.18 | Lowest |
| NCM811 | 462.52 | 4,887.41 | High |
| NCA | 491.84 | 4,878.15 | Higher |
| LCO | 545.11 | 5,841.18 | Highest |
The Safety Verdict: Why LFP Avoids Catastrophic Failure
The numbers in the table are clear. The LFP battery's maximum temperature of 239°C is less than half that of the LCO battery's 545°C. Even more, its heating rate is much, much lower: 0.18 °C/min compared to over 4,800 °C/min for the nickel-based chemistries.
This data leads to a very important conclusion. The maximum temperature reached by the LFP cell is well below the auto-ignition temperature of the organic electrolyte used in the battery. Its heating rate is so low that the event is more of a slow overheat than a runaway. In the study, a formal thermal runaway trigger temperature was not even detected for the LFP cell. This highlights its immense stability.
The word "failure" means something completely different for LFP compared to other chemistries. For an LFP battery, a failure under extreme abuse is a manageable overheating event. It may result in the venting of gases. For high-energy NMC and NCA batteries, a failure is a fast, high-temperature event. It can lead to fire and explosion because the cathode provides its own oxygen to fuel the fire. LFP chemistry fundamentally prevents this catastrophic outcome.
Practical Implications: The Trade-offs and Applications of LFP Batteries
The science behind LFP's safety leads to clear real-world pros and cons. Choosing LFP technology is an engineering decision. It prioritizes some performance features over others. This defines its best uses.
The Energy Density Compromise
The main trade-off for LFP's great safety is its lower energy density. LFP batteries typically store between 100-150 watt-hours per kilogram (Wh/kg). In comparison, high-energy NMC and NCA batteries can store 150-260 Wh/kg or more. So, for the same amount of energy storage, an LFP battery pack will be clearly larger and heavier.
Also, LFP cells have a lower nominal voltage of 3.2 volts, compared to the 3.6-3.7 volts of NMC or NCA cells. This difference requires more LFP cells to be connected in series to achieve the same voltage for a given application. This difference in energy density is a key factor in uses where weight and space are the top priorities, such as in long-range passenger electric vehicles or compact consumer electronics.
The Longevity and Cost Advantage
In return for lower energy density, LFP offers a much longer lifespan. LFP batteries can typically endure 3,000 to 6,000 full charge-discharge cycles, and sometimes more, before their capacity degrades significantly. This is much more than the 1,000 to 2,500 cycles expected from most NMC batteries.
Of course, in addition to the technical characteristics of the battery itself, proper use and maintenance are also crucial to maximizing the life of lithium-ion solar cells, ensuring that you get the most reliable performance over many years of use.
LFP batteries are also generally less expensive to make. Their cathode material uses iron and phosphate, which are common and low-cost raw materials. They do not contain cobalt. Cobalt is a metal that is expensive, has an unstable supply chain, and has ethical sourcing concerns. This cost advantage and their long life often lead to a much lower total cost of ownership over the battery's lifetime.
Ideal Use Cases for LFP Technology
The special profile of LFP—top safety and long life for lower energy density—makes it the better choice for a growing number of uses.
- Stationary Energy Storage: For home, business, and utility-scale systems that store solar or wind power, battery size is less important than safety, long life, and cost. LFP is the main chemistry in this market.
- Commercial and Fleet Electric Vehicles: For electric buses, delivery vans, forklifts, and other commercial vehicles, durability and operational safety are most important. These vehicles are used heavily every day, so LFP's long cycle life gives a clear economic benefit.
- Marine and Recreational Vehicles (RVs): In boats and RVs, the risk of fire is especially dangerous. LFP's natural safety is a key advantage. Its strength and long life are also highly valued in these demanding environments.
In the end, there is no single "best" battery chemistry for all uses. The market has grown to a point where different technologies are made for different needs. The choice between LFP and a higher-energy chemistry like NMC is a classic engineering trade-off. When designing large-scale grid energy storage facilities, engineers prioritize safety and cycle life. Therefore, lithium iron phosphate (LFP) batteries, which are known for their excellent safety, have become the first choice. An engineer designing a high-end smartphone will put compact size first, so they will choose a different chemistry. The fast growth of LFP technology directly shows the growth of industries where safety and long-term value are the most important needs.




Leave a comment
All comments are moderated before being published.
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.