The combination of lightweight aluminum frames and high-strength stainless steel fasteners is a global standard in the solar industry. This pairing provides the structural integrity needed for solar installations to withstand decades of environmental stress. Yet, a persistent question arises: does this pairing of dissimilar metals create a ticking time bomb for galvanic corrosion? This article separates myth from reality, explaining the science and providing practical solutions for ensuring the long-term durability of your solar array.
Understanding the Materials: A Tale of Two Metals
The choice of materials in a solar installation is deliberate, balancing cost, weight, strength, and longevity. Both aluminum and stainless steel offer unique properties that make them ideal for their respective roles.
Why Aluminum for Solar Frames?
Aluminum is the material of choice for most solar panel frames and mounting structures for several key reasons. It is lightweight, which simplifies transportation and reduces the structural load on rooftops. It also possesses excellent corrosion resistance due to a natural process called passivation. When exposed to air, aluminum forms a thin, hard layer of aluminum oxide on its surface, which protects the metal underneath from further corrosion. According to a report by the International Energy Agency, aluminum is a critical material used extensively in module frames and mounting structures for crystalline silicon PV technologies.
Why Stainless Steel for Fasteners?
Fasteners are the critical components holding the entire system together. They require exceptional strength to secure panels against wind, snow, and other mechanical stresses. Stainless steel, particularly grades like 304 and 316, provides this necessary strength along with superior corrosion resistance, making it the preferred material for bolts, nuts, and clamps in most solar installations.
The Galvanic Series: The Root of the Conflict
The concern about pairing these two metals originates from electrochemistry. Galvanic corrosion is a process that can occur when two different metals are in electrical contact in the presence of an electrolyte, like rainwater or coastal salt spray. In this scenario, the more 'active' (or less noble) metal becomes the anode and corrodes, while the less active (more noble) metal becomes the cathode and is protected. On the galvanic series, aluminum is more anodic than stainless steel, meaning it will corrode preferentially if the conditions are right.
Galvanic Corrosion in Solar Installations: Myth vs. Reality
The simple fact that aluminum and stainless steel are dissimilar metals does not automatically guarantee destructive corrosion. The risk is highly dependent on the specific environmental conditions of the installation site.

The Myth: Any Contact Leads to Immediate Failure
A common misconception is that simply bolting a stainless steel fastener to an aluminum frame will inevitably lead to rapid structural failure. This oversimplification ignores the critical role of the environment and other mitigating factors. In many dry, inland locations, solar arrays with this metal pairing have performed without issue for decades.
The Reality: It's All About the Environment
Significant galvanic corrosion requires three elements: two dissimilar metals, direct electrical contact, and a conductive electrolyte. The electrolyte is the key variable. In arid or minimally humid environments, there is often not enough moisture to facilitate the electrochemical reaction. The risk becomes substantial in high-risk zones:
- Coastal and Marine Environments: Salt spray from the ocean creates a highly conductive electrolyte, dramatically accelerating corrosion.
- Industrial Areas: Pollutants like sulfur dioxide and nitrous oxides can mix with atmospheric moisture to form acid rain, another potent electrolyte.
- Regions with High Humidity and Rainfall: Constant moisture provides a persistent pathway for the galvanic process to occur.
Assessing the Real-World Risk Factors
Beyond the simple presence of an electrolyte, other factors influence the rate and severity of galvanic corrosion on solar frames.
The Area Ratio Effect
The ratio of the cathode's surface area to the anode's surface area is a critical factor. A large cathode (noble metal) in contact with a small anode (active metal) is the worst-case scenario, as it concentrates the corrosive action on a small area. Fortunately, in solar installations, the situation is reversed: a small stainless steel fastener (cathode) is in contact with a large aluminum frame (anode). This large anode-to-cathode ratio spreads the corrosive effect over a much wider area, significantly slowing the rate of damage.
Passivation Layers: The Natural Defense
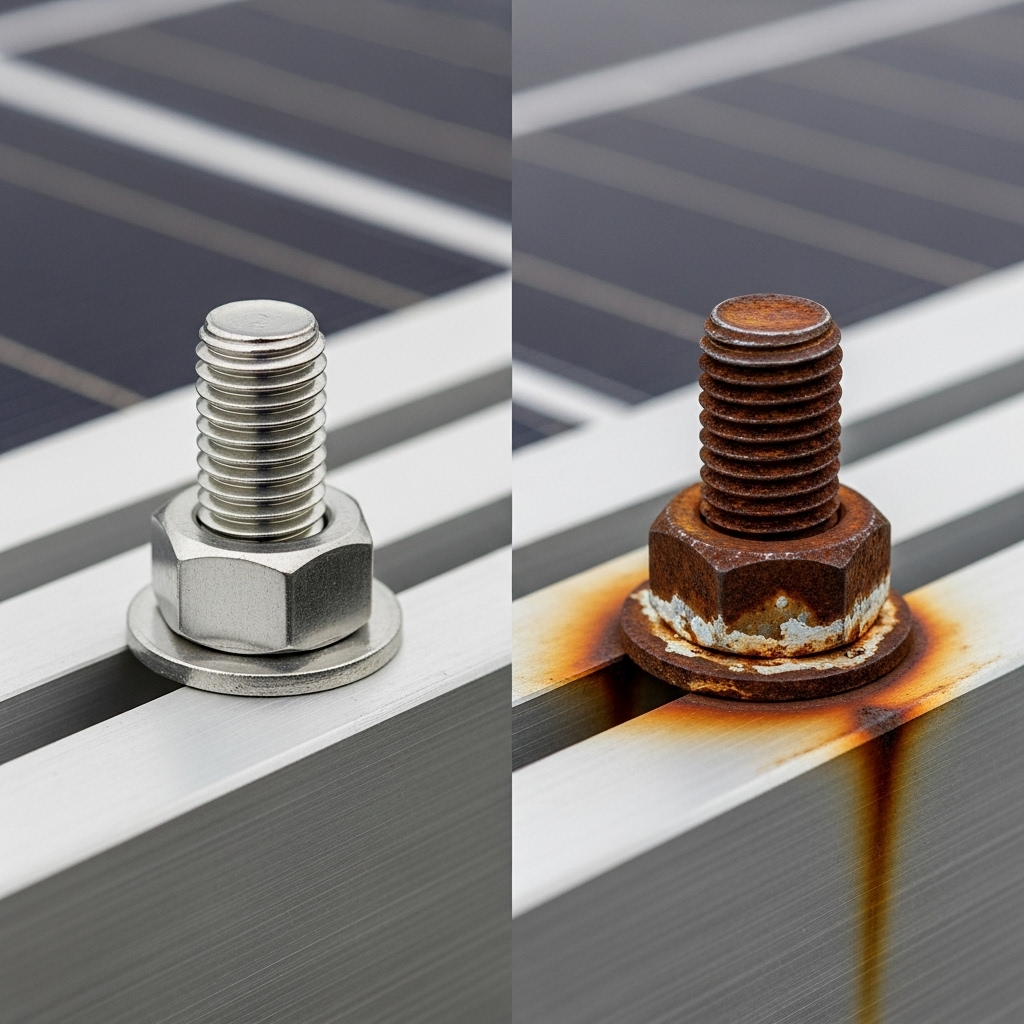
Both aluminum and stainless steel have protective oxide layers. Aluminum's oxide layer provides general protection, while stainless steel's chromium oxide layer makes it 'passive' or less reactive. However, these protective layers can be compromised. Chlorides, found in saltwater, are particularly effective at breaking down these passive films, exposing the raw metal and initiating corrosion.
Practical Solutions for Galvanic Isolation and Prevention
For installations in medium- to high-risk environments, proactive measures are essential to prevent aluminum stainless steel solar frame corrosion and ensure the system's longevity. The primary strategy is galvanic isolation.
Breaking the Circuit: Galvanic Isolation
The most effective method is to break the electrical circuit between the two metals. This is achieved by placing a non-conductive material between the aluminum frame and the stainless steel fastener. Common solutions include:
- Polymer Washers and Bushings: Placing a nylon or EPDM rubber washer under the head of a stainless steel bolt prevents direct contact with the aluminum surface.
- Specialized Gaskets and Pads: For mounting feet and other components, EPDM rubber pads can provide a durable, UV-resistant barrier.
- Protective Coatings: Anodizing the aluminum provides a thicker, more robust protective layer that acts as an insulator. If this layer is scratched during installation, however, the underlying aluminum can be exposed.
| Environment | Corrosion Risk | Recommended Action |
|---|---|---|
| Arid / Dry Inland | Low | Standard installation practices are often sufficient. Regular inspection is still advised. |
| Temperate / Moderate Humidity | Medium | Use of polymer washers or anodized components is recommended as a precaution. |
| Coastal / Marine / Industrial | High | Galvanic isolation is critical. Use stainless steel 316, EPDM gaskets, and polymer washers. Conduct frequent inspections. |
The Bigger Picture: Longevity and System Performance
Ensuring the mechanical stability of your panels is a cornerstone of long-term energy production. A compromised mounting system not only risks physical damage to the panels but can also lead to safety hazards. The integrity of every bolt and bracket is directly tied to the overall health and financial return of your solar energy system. This mechanical reliability is just as critical as the electrical efficiency of the components. For a complete perspective, understanding the metrics behind energy output is equally important; you can find a detailed analysis in the ultimate reference for solar storage performance.
A Balanced Approach to a Durable System
The pairing of aluminum frames and stainless steel fasteners is not an inherent flaw in solar system design; it is a robust and proven combination when applied correctly. The 'myth' of guaranteed failure gives way to the 'reality' that risk is entirely conditional. By understanding the science of galvanic corrosion, assessing the environmental risks, and implementing simple, effective galvanic isolation techniques, you can ensure your solar installation remains secure and productive for its entire expected lifespan. Proper material selection and thoughtful installation are the keys to building a truly resilient energy asset.
Frequently Asked Questions
Is it safe to use stainless steel bolts on an aluminum solar panel frame?
Yes, it is a common and generally safe industry practice, especially when proper precautions are taken in corrosive environments. In coastal, industrial, or highly humid areas, using galvanic isolation methods like nylon washers or EPDM gaskets is strongly recommended to prevent aluminum stainless steel fasteners solar mounting issues.
What are the first signs of galvanic corrosion on a solar rack?
The most common early sign is a white, powdery substance forming on the aluminum frame directly around the stainless steel fastener. This powder is aluminum oxide, which is the product of the aluminum (the anode) corroding due to the galvanic reaction.
How does galvanic isolation work?
Galvanic isolation involves inserting a non-conductive material, such as a plastic washer or a rubber pad, between the dissimilar metals. This barrier physically separates the aluminum and stainless steel, breaking the electrical circuit required for galvanic corrosion and stopping the destructive process before it can begin.
Are there alternatives to stainless steel fasteners for aluminum frames?
While stainless steel is preferred for its strength, alternatives include aluminum fasteners or specially coated steel fasteners. However, aluminum fasteners may not provide the same mechanical strength required to withstand high wind or snow loads. Coated steel fasteners can be effective, but their protective layer is vulnerable to being scratched during installation, which would expose the steel and create a new corrosion risk. For most applications, properly isolated stainless steel remains the most reliable choice.



Leave a comment
All comments are moderated before being published.
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.