The longevity of a solar panel installation depends heavily on the structural integrity of its mounting system. While solar panels are designed for decades of service, the racking that holds them is constantly exposed to the elements. Corrosion is a primary factor that can compromise the safety and lifespan of this critical infrastructure. Making an informed material choice based on solid data is fundamental to securing your energy investment for the long term.
Understanding Corrosion in Solar Racking Systems
Corrosion is an electrochemical process where metals degrade due to reactions with their environment. For solar racking, this process can weaken structural components, leading to potential system failure. Understanding the types of corrosion is the first step in prevention.
What is Corrosion and Why Does It Matter?
At its core, corrosion is the gradual destruction of materials by chemical or electrochemical reaction with their environment. In PV systems, this can mean rusted steel bolts or pitted aluminum rails. The consequences range from aesthetic issues to catastrophic structural failure, which can damage property and compromise the safety of the entire installation. A system's durability is a key factor in its overall return on investment.
Galvanic Corrosion: A Critical Concern
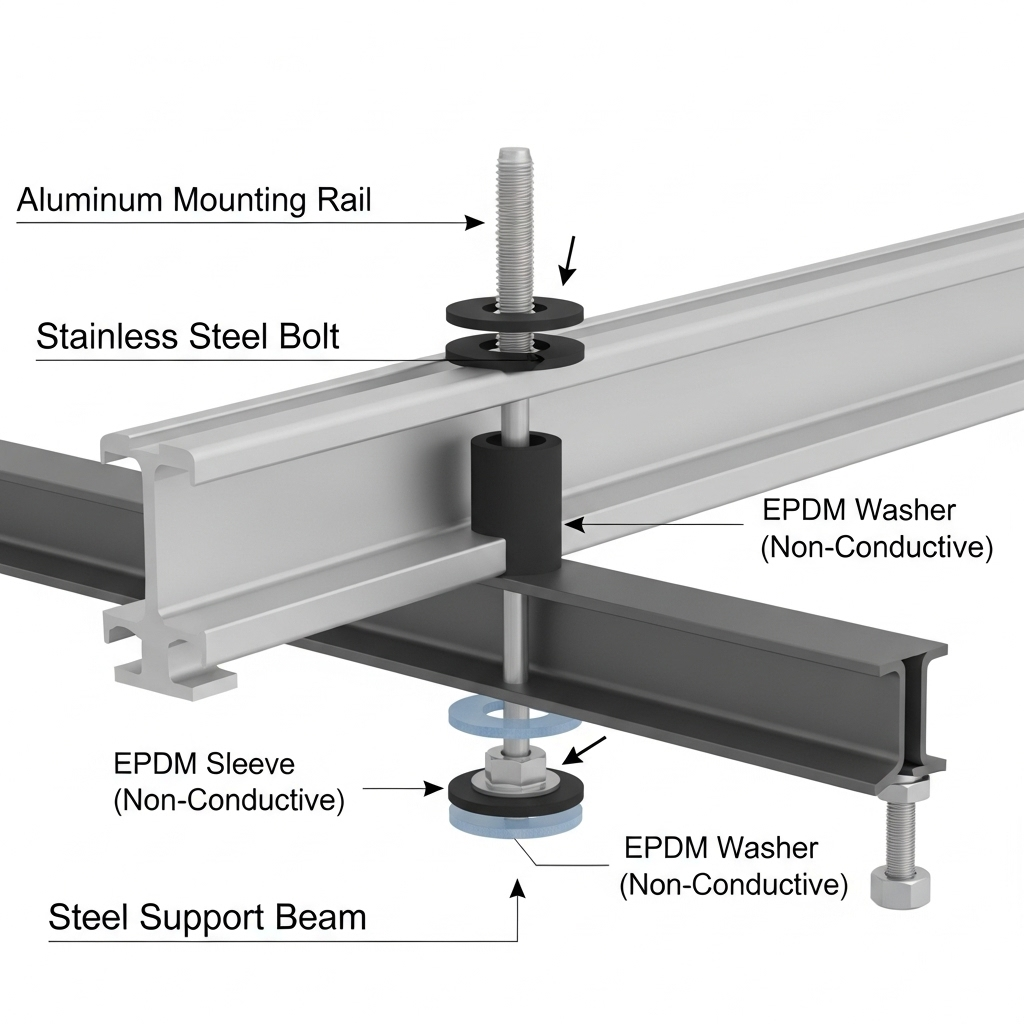
A specific and potent threat in solar installations is galvanic corrosion. This occurs when two different metals are in electrical contact in the presence of an electrolyte, like moisture. One metal becomes the anode and corrodes at an accelerated rate, while the other becomes the cathode and is protected. This is highly relevant for PV systems, where aluminum frames and racking are often secured with stainless steel fasteners. The greater the difference in electrochemical potential between the two metals, the faster the corrosion.
Common PV Racking Materials and Their Corrosion Resistance
The choice of material for PV racking is a balance of cost, strength, and durability. The three most common materials are aluminum, galvanized steel, and stainless steel, each with distinct properties affecting its performance in different environments.

Aluminum Alloys (e.g., 6000 series)
Aluminum is widely used for its high strength-to-weight ratio and inherent corrosion resistance. It naturally forms a protective oxide layer that prevents further degradation. Anodizing can enhance this protective layer, making it a suitable choice for many environments, including some coastal areas with moderate salinity.
Galvanized Steel
Galvanized steel offers excellent strength and is often more cost-effective than aluminum. Its corrosion protection comes from a layer of zinc, which acts as a sacrificial coating. The zinc corrodes first, protecting the steel underneath. The thickness of this coating, often designated by terms like 'G90', determines its lifespan, which can be significantly reduced in high-humidity, high-salinity, or polluted environments.
Stainless Steel (e.g., 304, 316)
Stainless steel provides the highest level of corrosion resistance due to its chromium content, which forms a robust passive film. While grade 304 is suitable for many applications, grade 316, with its added molybdenum, offers superior resistance to chlorides and is the preferred choice for marine or coastal installations. Though it has a higher initial cost, its longevity in harsh conditions can make it the most economical choice over the system's life.
Data-Backed Corrosion Rate Analysis
Selecting the right material requires looking beyond general characteristics and examining specific corrosion rate data. These rates are heavily influenced by the installation's environment.
Environmental Factors Influencing Corrosion
The primary drivers of corrosion are moisture, temperature, and atmospheric contaminants like salt (chlorides) and industrial pollutants (sulfur dioxide). An area with high humidity and salt spray, such as a coastline, will cause much faster degradation than a dry, rural inland location. The ISO 9223 standard classifies environments from C1 (very low corrosivity) to C5 (very high corrosivity) to help predict material lifespan.
Interpreting Corrosion Data
Corrosion rates are often measured in microns (µm) per year. This data allows for a quantitative comparison of how materials will perform over the expected 25-plus-year life of a PV system. The following table provides estimated corrosion rates based on environmental classifications.
| Material | Environment (ISO 9223) | Typical Corrosion Rate (µm/year) | Estimated Lifespan |
|---|---|---|---|
| Galvanized Steel (G90) | C2 (Rural) | 0.2 – 0.5 | 40-50+ years |
| Galvanized Steel (G90) | C3 (Urban/Light Industrial) | 0.5 – 1.0 | 15-25 years |
| Galvanized Steel (G90) | C4 (Coastal/Industrial) | 1.0 – 2.0 | 10-20 years |
| Galvanized Steel (G90) | C5 (Coastal/Offshore) | > 2.0 | 5-10 years |
| Aluminum (6000 Series) | C2-C3 | < 1.0 | 30+ years |
| Aluminum (6000 Series) | C4-C5 (Marine) | 1.0 - 2.5 | 15-30 years (Anodized) |
| Stainless Steel (316) | C2-C5 | < 0.1 | 50+ years |
Disclaimer: These values are estimates. Actual corrosion rates can vary based on specific site conditions, material quality, and installation practices.
Practical Strategies for Corrosion Mitigation
Beyond selecting the right base material, proper design and installation are critical for preventing premature corrosion and ensuring the system's longevity.
The Role of Galvanic Isolation
To prevent galvanic corrosion, it's essential to electrically isolate dissimilar metals. This can be achieved by using non-conductive polymer washers, pads, or sleeves between aluminum racking and stainless steel fasteners. This simple step breaks the electrical circuit required for galvanic corrosion to occur.
Selecting the Right Fasteners
Fasteners are often the first components to show signs of corrosion and can be a point of structural failure. Using high-quality stainless steel (grade 304 or 316) bolts, nuts, and washers is a common best practice for securing PV system components. Their compatibility and durability are crucial for the integrity of the entire array.
System Design and Maintenance
Good design includes ensuring proper drainage to prevent water from pooling on racking components. Regular inspections, at least annually, can help identify early signs of corrosion, allowing for corrective action before significant damage occurs. Just as a deep understanding of solar storage performance is key to a system's electrical efficiency, diligent maintenance is vital for its physical longevity and reliability.
A Forward-Looking Perspective on Material Durability
Investing in a solar energy system is a long-term commitment. The structural foundation of that system—the racking—should not be an afterthought. By using data to inform material selection, you can ensure the mounting structure lasts as long as the panels it supports. Matching the material's corrosion resistance to the specific environmental challenges of the installation site is the most effective way to protect your investment, ensure safety, and maximize the energy output for decades to come.
Frequently Asked Questions
How long will galvanized steel racking last in a coastal area?
In a coastal or marine environment (ISO category C4/C5), galvanized steel can have a significantly reduced lifespan, potentially lasting only 5 to 20 years, depending on the severity of salt exposure and humidity. Regular maintenance and thicker coatings can extend this, but stainless steel or properly anodized aluminum are often better long-term solutions in these settings.
Is stainless steel always the best option for PV racking?
While stainless steel offers superior corrosion resistance, it is also the most expensive option. In mild, dry, or rural environments (C1/C2), aluminum or even galvanized steel can provide sufficient protection for the system's lifespan at a lower initial cost. The 'best' option is always dependent on a cost-benefit analysis for the specific installation environment.
Can I use aluminum racks with steel bolts?
Using aluminum racks with stainless steel bolts is a very common practice in the solar industry. However, it creates a risk for galvanic corrosion. To mitigate this, it is crucial to use galvanic isolation methods, such as non-conductive washers or coatings, to separate the two metals, especially in moist or corrosive environments.




Leave a comment
All comments are moderated before being published.
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.