A solar energy system is a significant long-term investment, designed to operate for 25 years or more. The structural integrity of its mounting system is fundamental to achieving that lifespan. One of the most persistent threats to this integrity is galvanic corrosion, an electrochemical process that can weaken and destroy metal components, leading to potential system failure. Understanding and actively preventing this form of corrosion is crucial for ensuring the safety, durability, and performance of any solar installation.
The Science Behind Galvanic Corrosion in Solar Arrays
Galvanic corrosion, also known as bimetallic corrosion, is not simple rust. It is a specific electrochemical reaction that occurs when three conditions are met: two different metals are in electrical contact, and both are immersed in a conductive liquid known as an electrolyte. In a PV system, this electrolyte can be something as common as rain, dew, or humidity, especially in coastal areas with salt-laden air.
What Triggers This Electrochemical Reaction?
Every metal has a unique electrochemical potential. When two metals with different potentials are connected in an electrolyte, they form a galvanic cell, much like a battery. The less noble metal (the anode) gives up its electrons and corrodes at an accelerated rate, while the more noble metal (the cathode) is protected. The rate of corrosion is determined by the difference in potential between the two metals—the farther apart they are on the galvanic series, the more aggressive the corrosion.
Common Metal Pairings to Watch For in PV Systems
In solar installations, common materials like aluminum for racking and stainless steel for fasteners are often used together. While this combination is prevalent, it presents a risk for galvanic corrosion because aluminum is less noble than stainless steel. In the presence of moisture, the aluminum racking can corrode, sacrificing itself to protect the stainless steel bolts. Another problematic pairing is copper grounding components with aluminum frames, which can cause the aluminum to disintegrate over time.
Environmental Factors That Accelerate Corrosion
The surrounding environment plays a massive role in the speed of galvanic corrosion. High humidity provides a constant electrolyte, while salt spray in marine environments dramatically increases the conductivity of the moisture, accelerating the reaction. Industrial areas with pollutants like sulfur dioxide can also create more acidic rain, further increasing corrosion rates. Therefore, installations in coastal or industrial zones require heightened attention to corrosion prevention.
Strategic Material Selection: Your First Line of Defense
The most effective way to prevent galvanic corrosion is to eliminate one of the conditions that cause it. Thoughtful material selection from the outset is the most critical step in designing a durable and long-lasting PV mounting system.
Choosing Metals Close on the Galvanic Scale
A primary strategy is to select metals that are close together in the galvanic series. This minimizes the electrochemical potential difference between them, reducing the driving force of the corrosive reaction. For example, using aluminum fasteners with aluminum racking is ideal. When different metals are necessary, such as for fasteners requiring higher strength, choosing compatible combinations is key. According to research referenced in the Offshore wind energy: Patent insight report, cathodic protection is a key technology for preventing corrosion in harsh marine environments, highlighting the importance of electrochemical principles in system design.
| Metal Group | Common Materials | Compatibility Notes |
|---|---|---|
| Most Noble (Cathodic) | Stainless Steel (Passive), Titanium | Less likely to corrode. Can cause corrosion in less noble metals. |
| Intermediate | Bronze, Copper, Brass | Use with caution when paired with aluminum or zinc. |
| Least Noble (Anodic) | Aluminum, Galvanized Steel, Zinc | More likely to corrode when paired with more noble metals. |
The Role of Coatings and Finishes
Protective coatings provide a barrier that prevents the electrolyte from reaching the metal. Anodized aluminum, for instance, has a thick, durable layer of aluminum oxide that acts as an electrical insulator, reducing the risk of a galvanic reaction. Similarly, hot-dip galvanized steel is coated in a layer of zinc. The zinc not only provides a physical barrier but also acts as a sacrificial anode, corroding first to protect the underlying steel if the barrier is breached.
Assessing Material Compatibility for Fasteners and Racking
Always verify the material specifications for all components of a mounting system, from the rails and clamps down to the smallest nuts and bolts. Reputable manufacturers provide datasheets specifying material composition and compatibility. Adhering to standards like UL 2703 for mounting systems helps ensure that components have been tested for safety and durability.

Galvanic Isolation: Breaking the Corrosive Circuit
When using dissimilar metals is unavoidable, the next best strategy is galvanic isolation. This involves physically separating the metals to break the electrical circuit, thereby stopping the electrochemical reaction.
Using Non-Conductive Barriers
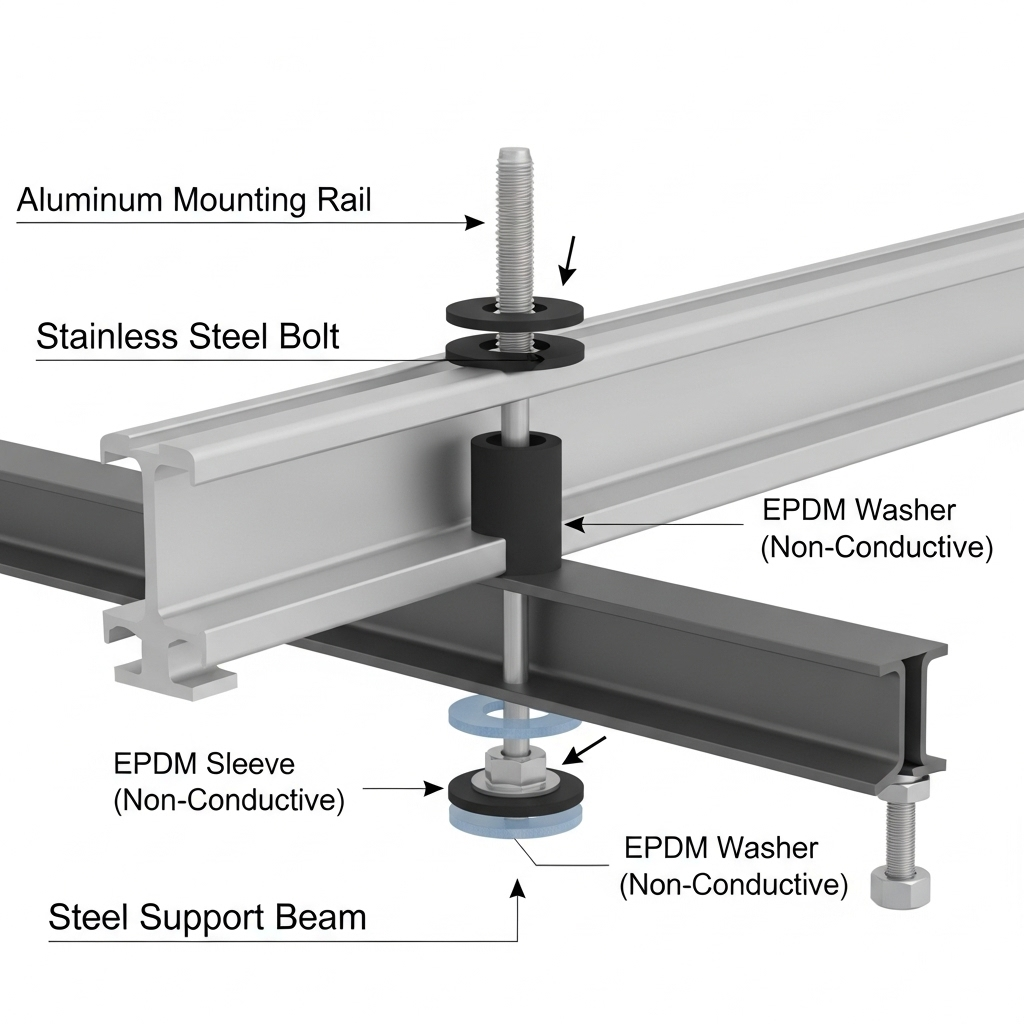
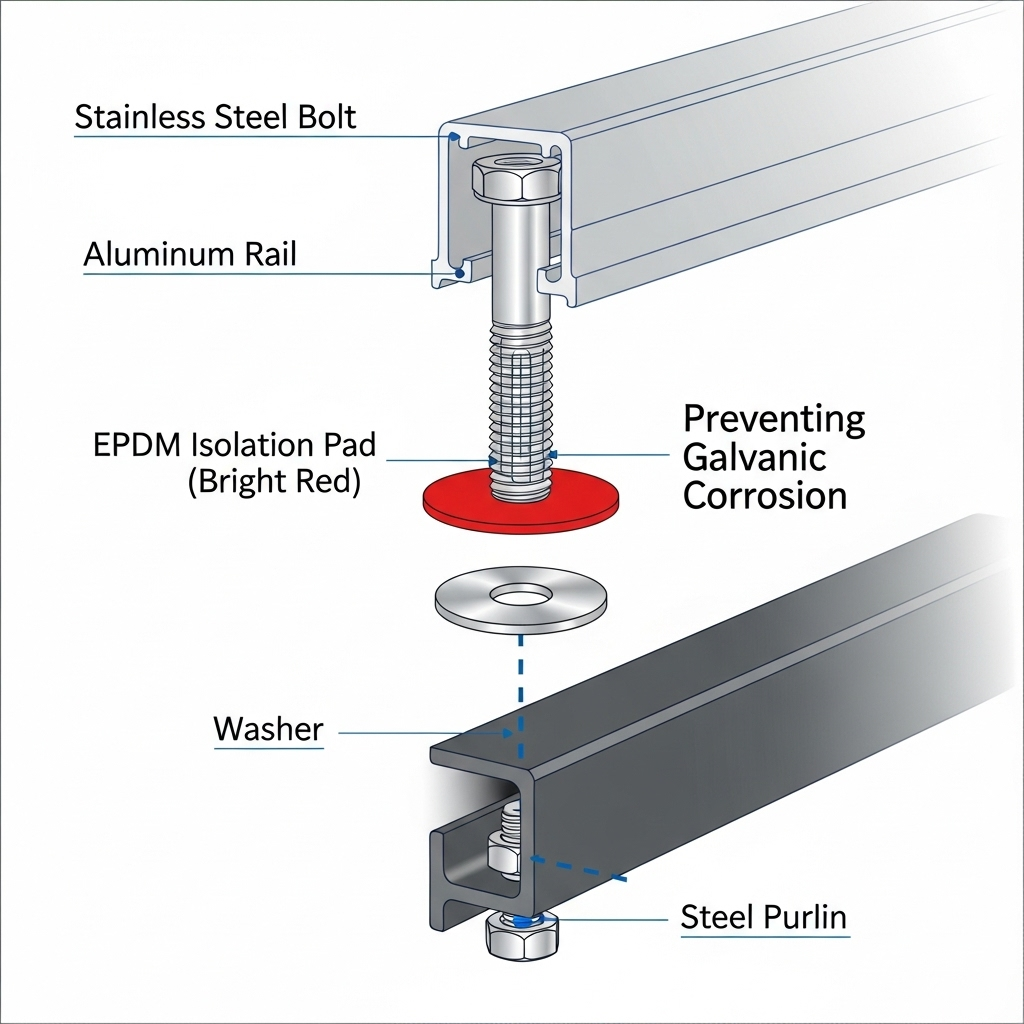
The most common method of galvanic isolation is using non-conductive washers, pads, or sleeves. These components are typically made from durable, weather-resistant materials like EPDM rubber, neoprene, or nylon. By placing a plastic washer between a stainless steel fastener and an aluminum rail, you prevent direct metal-to-metal contact, effectively halting the galvanic process.
Proper Installation Techniques for Isolation Components
The effectiveness of isolation components depends entirely on their correct installation. An installer must ensure that the barrier completely separates the two metals. For example, a specialized washer and bushing assembly might be needed to ensure a bolt is isolated from both the surface and the inside of the mounting hole. Leaving out these small but critical parts can render the entire prevention strategy useless.
Real-World Application: Coastal and Industrial Sites
In highly corrosive environments like coastal regions, galvanic isolation is not just a recommendation—it's a necessity. The combination of high humidity and salt spray creates a powerful electrolyte that can rapidly degrade mounting structures. Using marine-grade stainless steel or heavily anodized aluminum, combined with meticulous galvanic isolation at every connection point, is standard practice for ensuring a system's longevity in these challenging locations.
Long-Term Prevention and Maintenance Strategies
Preventing galvanic corrosion is an ongoing process that extends beyond the initial installation. A commitment to regular maintenance and inspection will protect the system for its entire operational life.
Regular Inspection Schedules
Periodic inspections are vital for catching corrosion early. System owners or maintenance professionals should look for signs of trouble, such as white, chalky residue on aluminum (a sign of oxidation), rust stains, or pitting on metal surfaces. Pay close attention to the connection points between different materials. Early detection allows for corrective action before significant structural damage occurs.
The Importance of Proper Drainage
Designs that allow water to pool create a persistent electrolyte bath, which is a recipe for accelerated corrosion. PV mounting systems should be designed and installed to promote water runoff. Keeping the components clean and dry minimizes the time they are exposed to an electrolyte, significantly slowing any potential corrosive reactions.
Linking System Health to Energy Storage Performance
The structural health of a PV array is directly linked to its electrical performance and, by extension, the performance of any connected energy storage system. A compromised mounting system can lead to shifting panels, damaged wiring, and reduced energy output. Maintaining the array's integrity is fundamental to achieving the consistent power generation needed for optimal battery charging and longevity. As detailed in the Ultimate Reference for Solar Storage Performance, stable input from your solar array is crucial for maximizing the efficiency and lifespan of your energy storage solution.
A Forward-Looking Approach to System Longevity
Protecting a solar investment means paying attention to every component, including the mounting system that holds it all together. Galvanic corrosion is a silent threat that can undermine the structural foundation of a PV array. By focusing on smart material selection, employing effective galvanic isolation techniques where needed, and committing to regular maintenance, you can ensure your solar mounting system remains robust and reliable. These proactive measures are essential for safeguarding the system's safety, maximizing energy production, and securing its financial return for decades.
Disclaimer: This article is for informational purposes only and does not constitute professional engineering or legal advice. Always consult with qualified professionals and adhere to local building codes and regulations for any solar installation.
Frequently Asked Questions
What are the first signs of galvanic corrosion on a PV mounting system?
The earliest signs include a white, powdery or chalky substance forming on aluminum components, especially around stainless steel fasteners. You may also see rust-colored stains, bubbling or peeling of coatings, and pitting on the metal surfaces where dissimilar metals are in contact.
Can you use stainless steel fasteners with aluminum racking?
Yes, but it must be done with caution. This is a very common practice in the solar industry. To prevent galvanic corrosion, it is essential to use a form of galvanic isolation, such as non-conductive polymer washers and sleeves, to create a barrier between the stainless steel and the aluminum. In less corrosive environments, the risk is lower, but isolation is always the safest practice.
How often should I inspect my solar mounts for corrosion?
A yearly inspection is a good baseline for most residential and commercial systems. However, for systems in highly corrosive environments, such as coastal or industrial areas, inspections should be performed every six months. Check for any signs of degradation, especially after severe weather events.
Are plastic or polymer-based isolation components durable in harsh weather?
High-quality isolation components are made from industrial-grade, UV-stabilized polymers like EPDM or nylon, which are designed to withstand decades of exposure to sunlight, extreme temperatures, and moisture without degrading. It is important to source these components from reputable suppliers who can verify their material specifications and durability ratings.



Leave a comment
All comments are moderated before being published.
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.