When selecting a battery for a home energy storage system, performance and cost are important factors. Yet, safety stands as the most critical consideration. Lithium-ion batteries are a family of different chemistries, and the term is often used broadly. However, distinct types exist, with Lithium Iron Phosphate (LiFePO4) emerging as a prominent choice for stationary storage. This raises a crucial question: Is a LiFePO4 battery genuinely safer than other common lithium-ion types, such as Nickel Manganese Cobalt (NMC)?
The answer lies in the fundamental differences in their chemistry, structure, and response to stress. While no battery is entirely without risk, the properties inherent to LiFePO4 provide a significant safety advantage, making it a highly reliable option for powering homes and off-grid applications.
The Foundation of Safety: A Look at Chemical and Structural Stability
The safety profile of any battery begins at the molecular level. The materials used in the cathode, one of the key components of a battery, largely determine its stability, especially under high temperatures or stress.
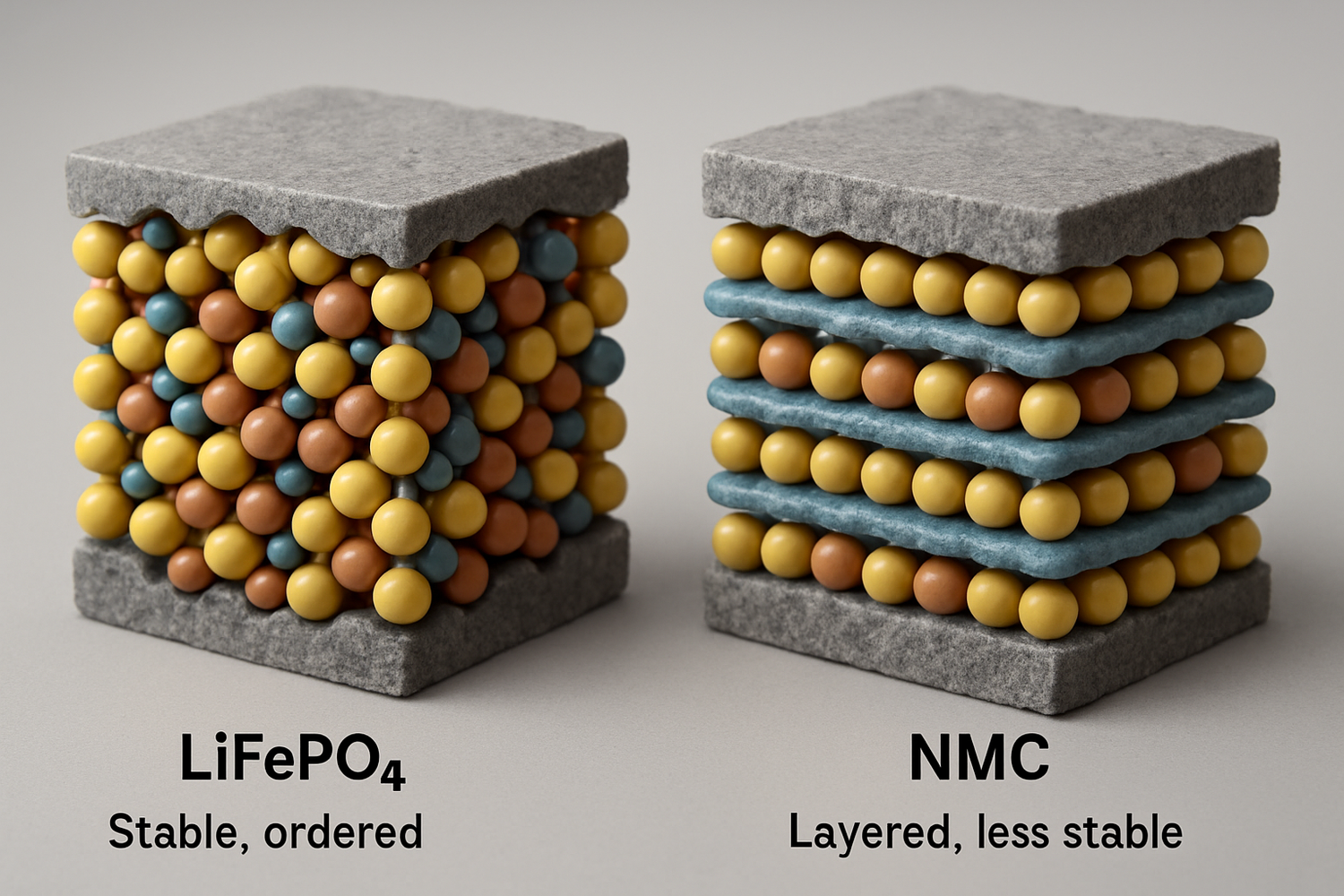
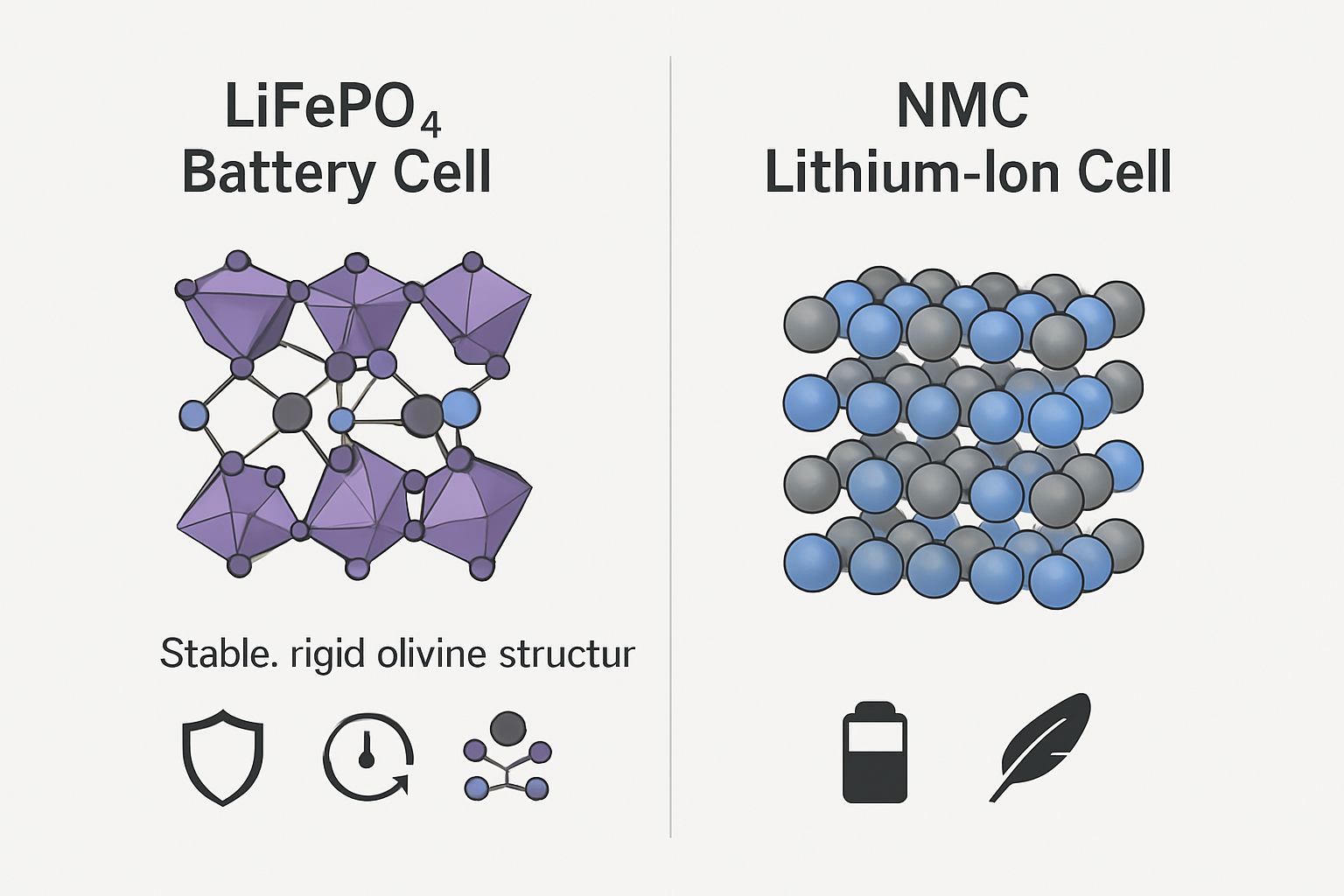
LiFePO4's Robust Olivine Structure
LiFePO4 batteries utilize a cathode material made of lithium iron phosphate, which has a crystal structure known as olivine. In this structure, the phosphorus and oxygen atoms are linked by powerful covalent bonds. This creates an exceptionally stable framework that is highly resistant to breaking down, even when the battery is subjected to physical damage or high temperatures. A key safety feature is that this structure does not release oxygen during decomposition, a critical factor in preventing thermal runaway.
Other Lithium-Ion Cathodes: NMC and LCO
In contrast, other popular lithium-ion chemistries like Nickel Manganese Cobalt (NMC) or Lithium Cobalt Oxide (LCO) use metal oxide cathodes. While these materials offer higher energy density, making them suitable for portable electronics and some electric vehicles where weight and space are primary concerns, their chemical structure is less stable. At elevated temperatures, these cathodes can begin to break down and release elemental oxygen. This oxygen can then act as a fuel, dramatically increasing the risk of a fire or explosion if a cell is compromised.
Thermal Runaway: The Critical Safety Benchmark
Thermal runaway is a dangerous condition where a battery enters an uncontrollable, self-heating state. An increase in temperature causes a chemical reaction that generates more heat, leading to a rapid and unstoppable cycle that can result in catastrophic failure. The temperature at which this process begins is a key indicator of a battery's safety.
Comparing Thermal Runaway Thresholds
A LiFePO4 battery possesses a significantly higher thermal runaway threshold compared to its NMC counterparts. Research shows that NMC cells can become unstable at temperatures as low as 160°C (320°F), while LiFePO4 cells remain stable up to approximately 230°C (446°F) or even higher in some tests. This wider margin of safety means a LiFePO4 battery can withstand more extreme temperatures and abuse before reaching a critical state. Once in thermal runaway, NMC cells can reach peak temperatures of 800°C, whereas LiFePO4 cells peak at a lower 620°C.
| Battery Chemistry | Typical Thermal Runaway Onset Temperature | Key Safety Characteristic |
|---|---|---|
| LiFePO4 (Lithium Iron Phosphate) | ~230-270°C | Does not release oxygen; stable olivine structure |
| NMC (Nickel Manganese Cobalt) | ~160-210°C | Can release oxygen, fueling thermal runaway |
The Role of the Battery Management System (BMS)
While chemistry provides the first line of defense, a high-quality Battery Management System (BMS) is an essential active safety feature in any lithium battery pack. The BMS continuously monitors cell voltage, current, and temperature. It protects the battery by preventing overcharging, over-discharging, and overheating—the primary triggers that can lead to thermal runaway. A well-designed BMS complements the inherent stability of LiFePO4 chemistry, creating a multi-layered safety system.
Real-World Performance Under Stress Conditions
A battery's true safety is revealed in how it responds to abuse and non-ideal conditions. In standardized tests that simulate real-world accidents, LiFePO4 technology consistently demonstrates superior resilience.
Response to Physical Damage
One of the most telling evaluations is the nail penetration test, which simulates an internal short circuit caused by physical damage. In numerous demonstrations, when a nail is driven through a fully charged NMC cell, it often results in a violent reaction, including fire and explosion. When the same test is performed on a LiFePO4 cell, it typically does not ignite or explode. It may release some vapor and heat up, but the reaction is far less volatile due to its stable chemistry.
Overcharging and Short-Circuit Tolerance
LiFePO4 batteries are also more tolerant of overcharging. Their strong chemical bonds resist the structural changes that can lead to failure in other lithium-ion types. While a BMS should prevent overcharging, the underlying chemistry of LiFePO4 provides an additional layer of protection should the primary systems fail. This stability is a core reason for its long cycle life, as the structure endures thousands of charge and discharge cycles with minimal degradation, a topic detailed in the ultimate reference for solar storage performance.
System-Level Safety and Official Certifications
Beyond the cell chemistry, the overall safety of an energy storage system depends on manufacturing quality and adherence to recognized standards. For stationary energy storage, safety is a priority for organizations like the U.S. Department of Energy, which actively supports research into next-generation battery safety.
The Importance of Quality Manufacturing
The safety advantages of LiFePO4 chemistry can be compromised by poor manufacturing. High-quality cells are built in controlled environments with pure materials to prevent contaminants that could cause internal shorts. Reputable manufacturers invest in stringent quality control to ensure every cell meets precise specifications, resulting in a reliable and safe final product.
Look for Recognized Safety Certifications
When choosing a LiFePO4 battery, look for certifications from recognized testing bodies like Underwriters Laboratories (UL). Standards such as UL1642 (Standard for Lithium Batteries) and UL1973 (Standard for Batteries for Use in Stationary Applications) involve rigorous testing for electrical, mechanical, and thermal safety. These certifications confirm that the battery has been independently verified to meet high safety requirements, providing confidence in its design and construction.
A Clearer Picture of Battery Safety
Based on its fundamental chemistry, superior thermal stability, and resilient performance under stress, the LiFePO4 battery is demonstrably safer than NMC and other common lithium-ion types. Its stable olivine structure, high thermal runaway threshold, and lack of oxygen release during decomposition collectively mitigate the most severe risks associated with lithium-ion technology. According to the International Energy Agency (IEA), LiFePO4 is already the fastest-growing chemistry for battery energy storage systems, partly due to its safety and longer cycle life. For home solar and off-grid energy storage, where safety and long-term reliability are paramount, the chemical and structural advantages of LiFePO4 make it the clear choice for achieving energy independence with peace of mind.
Frequently Asked Questions
Do LiFePO4 batteries ever catch fire?
While extremely rare, any battery can fail under specific circumstances, such as a severe manufacturing defect or extreme physical abuse that bypasses its safety mechanisms. However, the inherent chemical stability of LiFePO4 makes it far less prone to catching fire compared to other lithium-ion chemistries. The conditions required to cause a fire in a LiFePO4 battery are much more extreme.
Is the lower energy density of LiFePO4 a major drawback for home storage?
Not typically. Energy density refers to the amount of energy stored in a given volume or weight. While LiFePO4 batteries are slightly larger and heavier than NMC batteries of the same capacity, this is rarely a constraint for stationary applications like home energy storage. The significant gains in safety, longevity, and cost-effectiveness over the battery's lifespan usually outweigh the difference in size.
How does a BMS enhance the safety of a LiFePO4 battery?
A Battery Management System (BMS) acts as the brain of the battery pack. It protects the cells by preventing operations outside of their safe limits. Key functions include stopping the charge if the voltage gets too high (overcharge protection), cutting off power if the voltage gets too low (over-discharge protection), and monitoring temperature to prevent overheating. It ensures the battery operates safely and efficiently, maximizing its lifespan.


Leave a comment
All comments are moderated before being published.
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.